A new FDA report outlines how the pandemic has affected the agency's ability to conduct facility inspections and how it may be able to start resuming its normal inspection activities in the coming months.
In the report, Resiliency Roadmap for FDA Inspectional Oversight, regulators also acknowledge the significant backlog of domestic and foreign inspections, which is expected to persist throughout much of the next year.
We've highlighted the key takeaways from the report below.
Key Takeaways in FDA's New Inspections Report
1. Regulators quantified their inspection backlog.
FDA says it had planned to conduct 21,000 inspections across all regulated industries in FY2020 but only completed 13,000 before the pandemic. Regulators say those will roll over into FY2021, thereby creating a backlog. Currently, the agency puts the backlog numbers at:
- 2,426 medical device inspections;
- 857 human drug inspections;
- 110 biologics inspections, and
- 270 bioresearch monitoring inspections.
2. Regulators cite data showing an unprecedented slowdown in inspections beginning in March 2020.
Between March 2020 and March 2021, the FDA conducted roughly the same volume of inspections conducted over just one month in normal conditions:
- 155 total inspections of human drug facilities (49 mission-critical and 106 prioritized domestic)
- 80 total inspections of medical device facilities (8 mission-critical and 72 prioritized domestic);
- 63 total inspections of biologics facilities (10 mission-critical and 53 prioritized domestic), and
- 6 total inspections for animal drug facilities (0 mission-critical and 6 prioritized domestic).
3. Regulators formally acknowledge that delayed inspections are causing delayed decisions.
The agency states that delays in Pre-Approval Inspections have caused application decision delays.
4. Organizations can assess their risk of an immediate inspection using the “inspection priority” table.
See the excerpted table below. In general:
- The “mission critical” tier mostly includes for-cause inspections, inspections of manufacturers of essential or high-priority products, and products related to Covid-19.
- Products that fall under “Higher priority” include application approval activities for non-mission critical products and inspections of high-risk products.
- The “lower priority” tier includes inspections related to post-approval actions and routine surveillance.
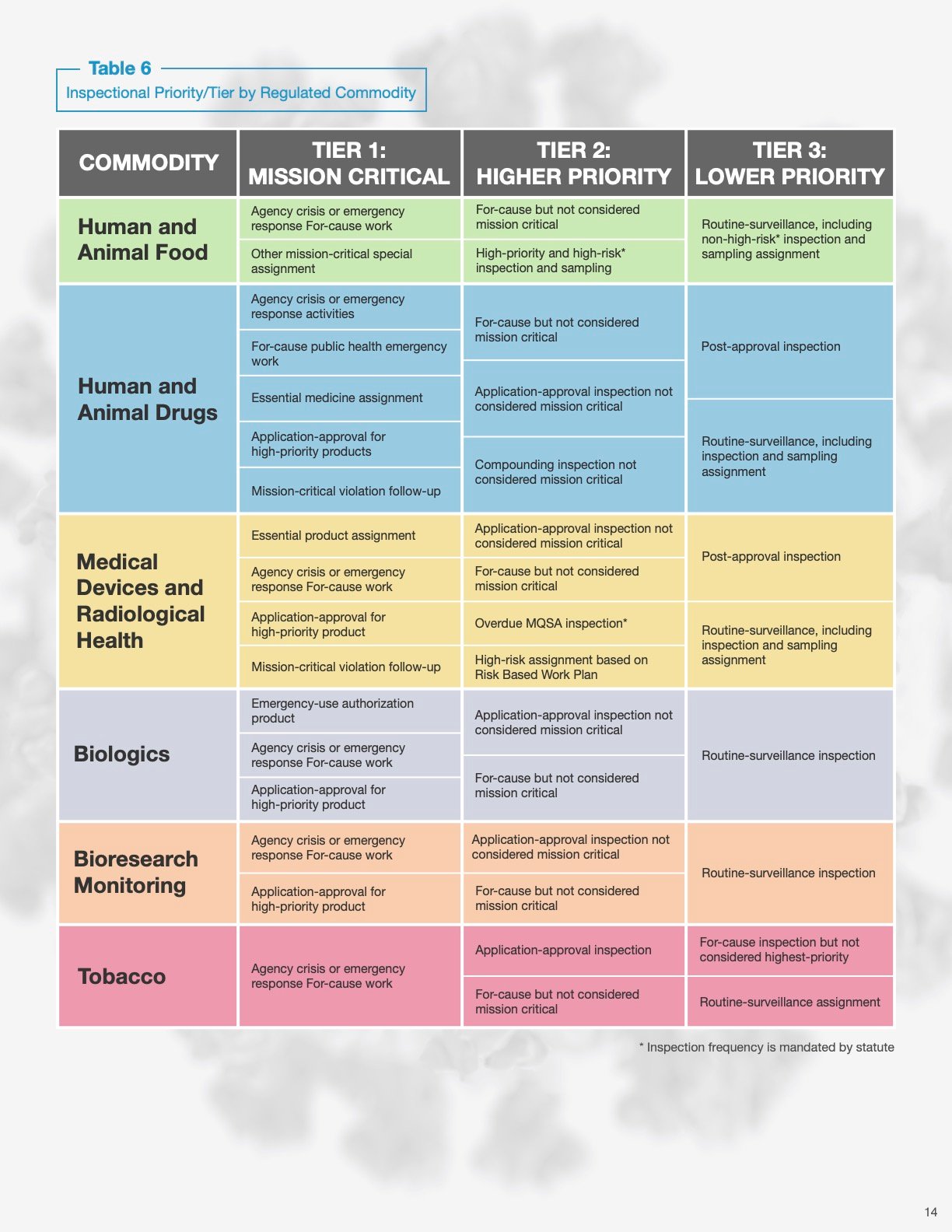
Source: Resiliency Roadmap for FDA Inspectional Oversight — fda.gov
From FDA's report:
"As Table 6 shows, mission-critical inspections will continue to be prioritized going forward. Routine surveillance inspections will continue to be conducted; however, the agency will prioritize higher-tiered needs. Thus, a longer interval between inspections will occur for lower-tiered inspection assignments as the agency adjusts to the impact of the COVID-19 pandemic. This means that postponed inspections will be prioritized based on risk and conducted over a longer period of time, ultimately increasing the amount of time between inspections of certain lower-risk facilities. The scenarios below estimate the routine surveillance work that could be accomplished, given certain assumptions. However, FDA recognizes the overall challenge presented by the volume of surveillance work, particularly related to lower-risk inspections that are nevertheless mandated to occur at certain frequencies (e.g., those mandated by FSMA, as discussed above). We will be exploring ways to manage this challenge moving forward."
 Facing a high-priority inspection, or simply want to ensure you're fully prepared for an FDA inspection whenever it may occur? Explore our auditing and remediation support services or get in touch with us to get the project or staffing support you need.
Facing a high-priority inspection, or simply want to ensure you're fully prepared for an FDA inspection whenever it may occur? Explore our auditing and remediation support services or get in touch with us to get the project or staffing support you need.
5. FDA Presents 3 Potential Scenarios for Returning to Inspection Normalcy
In its report, FDA also identifies three potential scenarios for returning to the normal pace of inspections.
- The base-case scenario: In this optimistic scenario, FDA expects to see a “gradual transition to standard operations” with a full transition expected to start in July 2021 and end in September 2021, at which point standard operations would resume. Importantly, this scenario assumes “no travel or establishment access restrictions,” which, given the pandemic's continued impact internationally, may not be the case for facilities outside of the U.S. Even under this scenario, FDA notes that only about 26 percent of the domestic medical product surveillance inspections that it planned to conduct in 2021 would be conducted.
- The best-case scenario: Here, FDA would resume standard operations as soon as May 2021. This scenario assumes no travel or access restrictions to facilities. Even in this best-case scenario, FDA estimates it would only get to half of the facility inspections it plans to conduct this year.
- The worst-case scenario: If new variants cause the pandemic to slide to new levels of severity and pose a significant risk to staff with significant restrictions on travel and facility access, FDA said it would “focus on oversight work most critical to its mission and limit inspection activities accordingly.” Regulators did not predict the number of inspections it may be able to conduct under this scenario, however, it noted that “alternative oversight tools” might be able to be used.
Join the thousands of companies executing their projects and ensuring inspection readiness with The FDA Group.
Now is the time to prepare for inspection readiness. Whether your organization could benefit from a mock FDA audit to identify and remediate issues before they’re detected by the FDA, or needs a resourcing solution—whether staff augmentation or FTE recruitment—now is the time to arrange for the support you need.
Explore our service areas and contact us today to get rapid access to the life science resources you need to fill specialized roles and execute projects on time and on budget. We offer a unique combination of experienced technical recruiting and project management capabilities to deliver both staffing/recruitment and project-based consulting solutions through your preferred engagement model.
GMP Auditing and COVID-19: A Guide to Remote Auditing and Workforce Recovery
Learn the challenges of remote auditing get strategies and best practices for overcoming them throughout each phase of the assessment process. This guide offers a playbook you can use to plan and host a successful remote audit.


